
Kelatorphan
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
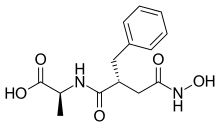
| Formula | C14H18N2O5 |
| Molar mass | 294.307 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Kelatorphan is a drug which acts as a powerful and complete inhibitor of nearly all of the enzymes responsible for catabolism of the endogenous enkephalins, including neutral endopeptidase (NEP), dipeptidyl peptidase III (DPP3), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE).[1][2][3] In mice, with the intracerebroventricular co-administration of a 50 μg dose of kelatorphan (this route is necessary because kelatorphan is incapable of crossing the blood-brain-barrier)[4] hence alongside exogenous [Met]enkephalin (ED50 approximately 10 ng), it potentiated the analgesic effects of the latter by 50,000 times.[1] Kelatorphan also displays potent antinociceptive effects alone,[5] and does not depress respiration, although at high doses it actually increases it.[4]

See also
References
- ^ a b Fournie-Zaluski MC, Chaillet P, Bouboutou R, Coulaud A, Cherot P, Waksman G, et al. (July 1984). "Analgesic effects of kelatorphan, a new highly potent inhibitor of multiple enkephalin degrading enzymes". European Journal of Pharmacology. 102 (3–4): 525–528. doi:10.1016/0014-2999(84)90575-2. PMID 6386492.
- ^ Yamamoto Y, Ono H, Ueda A, Shimamura M, Nishimura K, Hazato T (December 2002). "Spinorphin as an endogenous inhibitor of enkephalin-degrading enzymes: roles in pain and inflammation". Current Protein & Peptide Science. 3 (6): 587–599. doi:10.2174/1389203023380404. PMID 12470213. Archived from the original on 2013-04-14.
- ^ Robl JA, Trippodo Petrillo EW (5 September 1997). "Neutral Endopeptidase Inhibitors and Combined Inhibitors Neutral Endopeptidase and Angiotensin-Converting Enzyme". In van Zwieten PA, Greenlee WJ (eds.). Antihypertensive Drugs. CRC Press. p. 192. ISBN 978-90-5702-122-0. Retrieved 25 November 2011.
- ^ a b Boudinot E, Morin-Surun M, Foutz AS, Fournié-Zaluski M, Roques BP, Denavit-Saubié M (February 2001). "Effects of the potent analgesic enkephalin-catabolizing enzyme inhibitors RB101 and kelatorphan on respiration". Pain. 90 (1–2): 7–13. doi:10.1016/S0304-3959(00)00382-1. PMID 11166965. S2CID 26011241.
- ^ Kayser V, Fournie-Zaluski MC, Guilbaud G, Roques BP (September 1989). "Potent antinociceptive effects of kelatorphan (a highly efficient inhibitor of multiple enkephalin-degrading enzymes) systemically administered in normal and arthritic rats". Brain Research. 497 (1): 94–101. doi:10.1016/0006-8993(89)90974-8. PMID 2790459. S2CID 46293877.
See what we do next...
OR
By submitting your email or phone number, you're giving mschf permission to send you email and/or recurring marketing texts. Data rates may apply. Text stop to cancel, help for help.
Success: You're subscribed now !
