
Nonylphenol
| |
| Names | |
|---|---|
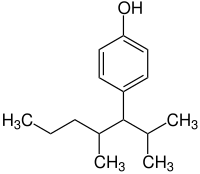
| IUPAC name
4-(2,4-dimethylheptan-3-yl)phenol
| |
| Other names
Phenol, nonyl-
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C15H24O | |
| Molar mass | 220.35 g/mol |
| Appearance | Light yellow viscous liquid with phenolic smell [1] |
| Density | 0.953 |
| Melting point | −8 to 2 °C (18 to 36 °F; 265 to 275 K) |
| Boiling point | 293 to 297 °C (559 to 567 °F; 566 to 570 K) |
| 6 mg/L (pH 7) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
low level endrocrine disruptor |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nonylphenols are a family of closely related organic compounds composed of phenol bearing a 9 carbon-tail. Nonylphenols can come in numerous structures, all of which may be considered alkylphenols. They are used in manufacturing antioxidants, lubricating oil additives, laundry and dish detergents, emulsifiers, and solubilizers.[2] They are used extensively in epoxy formulation in North America[3][4] but its use has been phased out in Europe.[5] These compounds are also precursors to the commercially important non-ionic surfactants alkylphenol ethoxylates and nonylphenol ethoxylates, which are used in detergents, paints, pesticides, personal care products, and plastics. Nonylphenol has attracted attention due to its prevalence in the environment and its potential role as an endocrine disruptor and xenoestrogen, due to its ability to act with estrogen-like activity.[6] The estrogenicity and biodegradation heavily depends on the branching of the nonyl sidechain.[7][8][9] Nonylphenol has been found to act as an agonist of the GPER (GPR30).[10]

Structure and basic properties
Nonylphenols fall into the general chemical category of alkylphenols.[11] The structure of NPs may vary. The nonyl group can be attached to the phenol ring at various locations, usually the 4- and, to lesser extent, the 2-positions, and can be either branched or linear. A branched nonylphenol, 4-nonylphenol, is the most widely produced and marketed nonylphenol.[12] The mixture of nonylphenol isomers is a pale yellow liquid, although the pure compounds are colorless. The nonylphenols are moderately soluble in water [12] but soluble in alcohol.

Nonylphenol arises from the environmental degradation of nonylphenol ethoxylates, which are the metabolites of commercial detergents called alkylphenol ethoxylates. NPEs are a clear to light orange color liquid. Nonylphenol ethoxylates are nonionic in water, which means that they have no charge. Because of this property they are used as detergents, cleaners, emulsifiers, and a variety of other applications. They are amphipathic, meaning they have both hydrophilic and hydrophobic properties, which allows them to surround non-polar substances like oil and grease, isolating them from water.[2]

Production
Nonylphenol can be produced industrially, naturally, and by the environmental degradation of alkylphenol ethoxylates. Industrially, nonylphenols are produced by the acid-catalyzed alkylation of phenol with a mixture of nonenes. This synthesis leads to a very complex mixture with diverse nonylphenols.[13][14][15] Theoretically there are 211 constitutional isomers and this number rise to 550 isomers if we take the enantiomers into account.[7] To make NPEs, manufacturers treat NP with ethylene oxide under basic conditions.[12] Since its discovery in 1940, nonylphenol production has increased exponentially, and between 100 and 500 million pounds of nonylphenol are produced globally every year,[12][16] meeting the definition of High Production Volume Chemicals.

Nonylphenols are also produced naturally in the environment. One organism, the velvet worm, produces nonylphenol as a component of its defensive slime. The nonylphenol coats the ejection channel of the slime, stopping it from sticking to the organism when it is secreted. It also prolongs the drying process long enough for the slime to reach its target.[17]

Another surfactant called nonoxynol, which was once used as intravaginal spermicide and condom lubricant, was found to metabolize into free nonylphenol when administered to lab animals.[11]

Applications
Nonylphenol is used in manufacturing antioxidants, lubricating oil additives, laundry and dish detergents, emulsifiers, and solubilizers.[2] It can also be used to produce tris(4-nonyl-phenyl) phosphite (TNPP), which is an antioxidant used to protect polymers, such as rubber, Vinyl polymers, polyolefins, and polystyrenics in addition to being a stabilizer in plastic food packaging. Barium and calcium salts of nonylphenol are also used as heat stabilizers for polyvinyl chloride (PVC).[18] Nonylphenol is also often used an intermediate in the manufacture of the non-ionic surfactants nonylphenol ethoxylates, which are used in detergents, paints, pesticides, personal care products, and plastics. Nonylphenol and nonylphenol ethoxylates are only used as components of household detergents outside of Europe.[2] Nonyl Phenol, is used in many epoxy formulations mainly in North America.

Prevalence in the environment
Nonylphenol persists in aquatic environments and is moderately bioaccumulative. It is not readily biodegradable, and it can take months or longer to degrade in surface waters, soils, and sediments. Nonbiological degradation is negligible.[6] Nonylphenol is partially removed during municipal wastewater treatment due to sorption to suspended solids and biotransformation.[19][20] Many products that contain nonylphenol have "down-the-drain" applications, such as laundry and dish soap, so the contaminants are frequently introduced into the water supply. In sewage treatment plants, nonylphenol ethoxylate degrades into nonylphenol, which is found in river water and sediments as well as soil and groundwater.[21] Nonylphenol photodegrades in sunlight, but its half-life in sediment is estimated to be more than 60 years. Although the concentration of nonylphenol in the environment is decreasing, it is still found at concentrations of 4.1 μg/L in river waters and 1 mg/kg in sediments.[2]

A major concern is that contaminated sewage sludge is frequently recycled onto agricultural land. The degradation of nonylphenol in soil depends on oxygen availability and other components in the soil. Mobility of nonylphenol in soil is low.[2]

Bioaccumulation is significant in water-dwelling organisms and birds, and nonylphenol has been found in internal organs of certain animals at concentrations of 10 to 1,000 times greater than the surrounding environment.[6] Due to this bioaccumulation and persistence of nonylphenol, it has been suggested that nonylphenol could be transported over long distances and have a global reach that stretches far from the site of contamination.[22]

Nonylphenol is not persistent in air, as it is rapidly degraded by hydroxyl radicals.[6]

Environmental hazards
Nonylphenol is considered to be an endocrine disruptor due to its ability to mimic estrogen and in turn disrupt the natural balance of hormones in affected organisms.[7][8][9][23][24] The effect is weak because nonylphenols are not very close structural mimics of estradiol, but the levels of nonylphenol can be sufficiently high to compensate.


The effects of nonylphenol in the environment are most applicable to aquatic species. Nonylphenol can cause endocrine disruption in fish by interacting with estrogen receptors and androgen receptors. Studies report that nonylphenol competitively displaces estrogen from its receptor site in rainbow trout.[25] It has much less affinity for the estrogen receptor than estrogen in trout (5 x 10−5 relative binding affinity compared to estradiol) making it 100,000 times less potent than estradiol.[25][26] Nonylphenol causes the feminization of aquatic organisms, decreases male fertility, and decreases survival in young fish.[2] Studies show that male fish exposed to nonylphenol have lower testicular weight.[25] Nonylphenol can disrupt steroidogenesis in the liver. One function of endogenous estrogen in fish is to stimulate the liver to make vitellogenin, which is a phospholipoprotein.[25] Vitellogenin is released by the maturing female and sequestered by developing oocytes to produce the egg yolk.[25] Males do not normally produce vitellogenin, but when exposed to nonylphenol they produce similar levels of vitellogenin to females.[25] The concentration needed to induce vitellogenin production in fish is 10 ug/L for NP in water.[25] Nonylphenol can also interfere with the level of FSH (follicle-stimulating hormone) being released from the pituitary gland. Concentrations of NP that inhibit reproductive development and function in fish also damages kidneys, decreases body weight, and induces stressed behavior.[27]

Human health hazards
Alkylphenols like nonylphenol and bisphenol A have estrogenic effects in the body. They are known as xenoestrogens.[28] Estrogenic substances and other endocrine disruptors are compounds that have hormone-like effects in both wildlife and humans. Xenoestrogens usually function by binding to estrogen receptors and acting competitively against natural estrogens. Nonylphenol has been shown to mimic the natural hormone 17β-estradiol, and it competes with the endogenous hormone for binding with the estrogen receptors ERα and ERβ.[2] Nonylphenol was discovered to have hormone-like effects by accident because it contaminated other experiments in laboratories that were studying natural estrogens that were using polystyrene tubes.[11]

Effects in pregnant women
Subcutaneous injections of nonylphenol in late pregnancy causes the expression of certain placental and uterine proteins, namely CaBP-9k, which suggest it can be transferred through the placenta to the fetus. It has also been shown to have a higher potency on the first trimester placenta than the endogenous estrogen 17β-estradiol. In addition, early prenatal exposure to low doses of nonylphenol cause an increase in apoptosis (programmed cell death) in placental cells. These “low doses” ranged from 10−13-10−9 M, which is lower than what is generally found in the environment.[29]

Nonylphenol has also been shown to affect cytokine signaling molecule secretions in the human placenta. In vitro cell cultures of human placenta during the first trimester were treated with nonylphenol, which increase the secretion of cytokines including interferon gamma, interleukin 4, and interleukin 10, and reduced the secretion of tumor necrosis factor alpha. This unbalanced cytokine profile at this part of pregnancy has been documented to result in implantation failure, pregnancy loss, and other complications.[29]

Effects on metabolism
Nonylphenol has been shown to act as an obesity enhancing chemical or obesogen, though it has paradoxically been shown to have anti-obesity properties.[30] Growing embryos and newborns are particularly vulnerable when exposed to nonylphenol because low-doses can disrupt sensitive processes that occur during these important developmental periods.[31] Prenatal and perinatal exposure to nonylphenol has been linked with developmental abnormalities in adipose tissue and therefore in metabolic hormone synthesis and release (Merrill 2011). Specifically, by acting as an estrogen mimic, nonylphenol has generally been shown to interfere with hypothalamic appetite control.[30] The hypothalamus responds to the hormone leptin, which signals the feeling of fullness after eating, and nonylphenol has been shown to both increase and decrease eating behavior by interfering with leptin signaling in the midbrain.[30] Nonylphenol has been shown mimic the action of leptin on neuropeptide Y and anorectic POMC neurons, which has an anti-obesity effect by decreasing eating behavior. This was seen when estrogen or estrogen mimics were injected into the ventromedial hypothalamus.[32] On the other hand, nonylphenol has been shown to increase food intake and have obesity enhancing properties by lowering the expression of these anorexigenic neurons in the brain.[33] Additionally, nonylphenol affects the expression of ghrelin: an enzyme produced by the stomach that stimulates appetite.[34] Ghrelin expression is positively regulated by estrogen signaling in the stomach, and it is also important in guiding the differentiation of stem cells into adipocytes (fat cells). Thus, acting as an estrogen mimic, prenatal and perinatal exposure to nonylphenol has been shown to increase appetite and encourage the body to store fat later in life.[35] Finally, long-term exposure to nonylphenol has been shown to affect insulin signaling in the liver of adult male rats.[36]

Cancer
Nonylphenol exposure has also been associated with breast cancer.[2] It has been shown to promote the proliferation of breast cancer cells, due to its agonistic activity on ERα (estrogen receptor α) in estrogen-dependent and estrogen-independent breast cancer cells. Some argue that nonylphenol's suggested estrogenic effect coupled with its widespread human exposure could potentially influence hormone-dependent breast cancer disease.[37]

Human exposure and breakdown
Exposure
Diet seems the most significant source of exposure of nonylphenol to humans. For example, food samples were found with concentrations ranging from 0.1 to 19.4 μg/kg in a diet survey in Germany and a daily intake for an adult were calculated to be 7.5 μg/day.[38] Another study calculated a daily intake for the more exposed group of infants in the range of 0.23-0.65 μg per kg bodyweight per day.[39] In Taiwan, nonylphenol concentrations in food ranged from 5.8 to 235.8 μg/kg. Seafood in particular was found to have a high concentration of nonylphenol.[40]

One study conducted in Italian women showed that nonylphenol was one of the highest contaminants at a concentration of 32 ng/mL in breast milk when compared to other alkyl phenols, such as octylphenol, nonylphenol monoethoxylate, and two octylphenol ethoxylates. The study also found a positive correlation between fish consumption and the concentration of nonylphenol in breast milk.[40] This is a large problem because breast milk is the main source of nourishment for newborns, who are in early stages of development where hormones are very influential. Elevated levels of endocrine disruptors in breast milk have been associated with negative effects on neurological development, growth, and memory function.

Drinking water does not represent a significant source of exposure in comparison to other sources such as food packing materials, cleaning products, and various skin care products. Concentrations of nonylphenol in treated drinking water varied from 85 ng/L in Spain to 15 ng/L in Germany.[2]

Microgram amounts of nonylphenol have also been found in the saliva of patients with dental sealants.[37]

Breakdown
When humans orally ingest nonylphenol, it is rapidly absorbed in the gastrointestinal tract. The metabolic pathways involved in its degradation are thought to involve glucuronide and sulfate conjugation, and the metabolites are then concentrated in fat. There is inconsistent data on bioaccumulation in humans, but nonylphenol has been shown to bioaccumulate in water-dwelling animals and birds. Nonylphenol is excreted in feces and in urine.[6]

Analytics
There are standard GC-MS and HPLC protocols for the detection of nonylphenols within environmental sample matrices such as foodstuffs, drinking water and biological tissue.[41][42] Industrially produced nonylphenol (the source most likely to be found in the environment) contains a mixture of structural isomers,[43] and while these protocols are able to detect this mixture, they are typically unable to resolve the individual nonylphenol isomers within it. However, a methodological study has indicated that better isomeric resolution can be achieved in bulk nonylphenol samples using a GC-MS/MS (tandem mass-analyzer) system,[44] suggesting that this technique could also improve the resolution of nonylphenol isomers in environmental sample analyses; further improvements in the resolution of nonylphenol isomers have been achieved through the use of two-dimensional GC at the separation stage, as part of a GC x GC-TOF-MS system.[45]

In contrast to environmental sample analyses, synthetic studies of nonylphenols have more control over sample state, concentration and preparation, simplifying the use of powerful structural identification techniques like NMR - capable of identifying the individual nonylphenol isomers.[46] In a preliminary investigation of the relationship between nonylphenol sidechain branching patterns and estrogenic potential, the authors identified 211 possible structural isomers of p-nonylphenol alone, which expanded to 550 possible p-nonylphenol compounds when taking chiral C-atoms into consideration.[47] Because stereochemical factors are thought to contribute to the biological activity of nonylphenols, analytical techniques sensitive to chirality, such as enantioselective HPLC and certain NMR protocols, are desirable in order to further study these relationships.[48][49][50]

Regulation
The production and use of nonylphenol and nonylphenol ethoxylates is prohibited for certain situations in the European Union due to its effects on health and the environment.[2][51] In Europe, due to environmental concerns, they also have been replaced by more expensive alcohol ethoxylates, which are less problematic for the environment due to their ability to degrade more quickly than nonylphenols. The European Union has also included NP on the list of priority hazardous substances for surface water in the Water Framework Directive. They are now implementing a drastic reduction policy of NP's in surface waterways. The Environmental quality standard for NP was proposed to be 0.3 ug/L.[2] In 2013 nonylphenols were registered on the REACH candidate list.

In the US, the EPA set criteria which recommends that nonylphenol concentration should not exceed 6.6 ug/L in fresh water and 1.7 ug/L in saltwater.[52] In order to do so, the EPA is supporting and encouraging a voluntary phase-out of nonylphenol in industrial laundry detergents. Similarly, the EPA is documenting proposals for a "significant new use" rule, which would require companies to contact the EPA if they decided to add nonylphenol to any new cleaning and detergent products. They also plan to do more risk assessments to ascertain the effects of nonylphenol on human health and the environment.

In other Asian and South American countries nonylphenol is still widely available in commercial detergents, and there is little regulation.[52]

References
- ^ Record of Nonylphenol, mixed isomers in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 6 April 2011.
- ^ a b c d e f g h i j k l Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E. (2008). "Nonylphenol in the Environment: A Critical Review on Occurrence, Fate, Toxicity and Treatment in Wastewaters". Environment International. 34 (7): 1033–049. doi:10.1016/j.envint.2008.01.004. PMID 18282600.
- ^ "Epoxy Nonyl Phenol Alert is it in your epoxy?". www.epoxyproducts.com. Retrieved 2021-03-18.
- ^ "para nonyl phenol" (PDF). squarespace.
- ^ "No, no, nonyl(phenol)". Healthy Building Network. Retrieved 2021-03-18.
- ^ a b c d e Mergel, Maria. "Nonylphenol and Nonylphenol Ethoxylates." Toxipedia.org. N.p., 1 Nov. 2011. Web. 27 Apr. 2014.
- ^ a b c Guenther, Klaus; Kleist, Einhard; Thiele, Bjoern (2005-12-10). "Estrogen-active nonylphenols from an isomer-specific viewpoint: a systematic numbering system and future trends". Analytical and Bioanalytical Chemistry. 384 (2): 542–546. doi:10.1007/s00216-005-0181-8. ISSN 1618-2642. PMID 16341851. S2CID 39833642.
- ^ a b Gabriel FL, Routledge EJ, Heidelberger A, Rentsch D, Guenther K, Giger W, et al. (2008). "Isomer-specific degradation and endocrine disrupting activity of nonylphenols" (PDF). Environ Sci Technol. 42 (17): 6399–408. Bibcode:2008EnST...42.6399G. doi:10.1021/es800577a. PMID 18800507.
- ^ a b Lu, Zhijiang; Gan, Jay (2014-01-21). "Isomer-Specific Biodegradation of Nonylphenol in River Sediments and Structure-Biodegradability Relationship". Environmental Science & Technology. 48 (2): 1008–1014. Bibcode:2014EnST...48.1008L. doi:10.1021/es403950y. ISSN 0013-936X. PMID 24345275.
- ^ Prossnitz, Eric R.; Barton, Matthias (2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. ISSN 0303-7207. PMC 4040308. PMID 24530924.
- ^ a b c Sonnenschein, Carlos; Soto, Ana M. (1998). "An Updated Review of Environmental Estrogen and Androgen Mimics and Antagonists". The Journal of Steroid Biochemistry and Molecular Biology. 65 (1–6): 143–50. doi:10.1016/s0960-0760(98)00027-2. PMID 9699867. S2CID 16158451.
- ^ a b c d EPA. 2010. Nonylphenol (NP) and Nonylphenal Ethoxylates (NPEs) Action Plan. February, 2014.
- ^ Thiele, Bjoern; Heinke, Volkmar; Kleist, Einhard; Guenther, Klaus (2004-06-01). "Contribution to the Structural Elucidation of 10 Isomers of Technical p-Nonylphenol". Environmental Science & Technology. 38 (12): 3405–3411. Bibcode:2004EnST...38.3405T. doi:10.1021/es040026g. ISSN 0013-936X. PMID 15260341.
- ^ Ruß, Alexander S.; Vinken, Ralph; Schuphan, Ingolf; Schmidt, Burkhard (2005-09-01). "Synthesis of branched para-nonylphenol isomers: Occurrence and quantification in two commercial mixtures". Chemosphere. 60 (11): 1624–1635. Bibcode:2005Chmsp..60.1624R. doi:10.1016/j.chemosphere.2005.02.046. PMID 16083769.
- ^ Wheeler, Todd F.; Heim, John R.; LaTorre, Maria R.; Janes, A. Blair (1997-01-01). "Mass Spectral Characterization of p-Nonylphenol Isomers Using High-Resolution Capillary GC—MS". Journal of Chromatographic Science. 35 (1): 19–30. doi:10.1093/chromsci/35.1.19. ISSN 0021-9665.
- ^ Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef; Garbe, Dorothea; Paulus, Wilfried (2000). Phenol Derivatives. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
{{cite book}}:|journal=ignored (help) - ^ Benkendorff, K.; Beardmore, K.; Gooley, A. A.; Packer, N. H.; Tait, N. N. (1999). "Characterisation of the slime gland secretion from the peripatus, Euperipatoides kanangrensis (Onychophora: Peripatopsidae)". Comparative Biochemistry and Physiology B. 124 (4): 457–465. doi:10.1016/S0305-0491(99)00145-5.
- ^ Nonylphenol and nonylphenol ethoxylates action plan. U.S. Environmental Protection Agency (EPA). August 2010.
- ^ Samaras et al. 2013. Fate of selected pharmaceuticals and synthetic endocrine disruptive compounds during wastewater treatment and sludge anaerobic digestion. Journal of Hazardous Materials, vol. 244-245, January 2013, p. 259-267.Samaras et al., 2013
- ^ Stasinakis et al., 2013. Contribution of primary and secondary treatment on the removal of benzothiazoles, benzotriazoles, endocrine disruptors, pharmaceuticals and perfluorinated compounds in a sewage treatment plant. Sci. Total Environ. Vol. 463-464, October 2013, p. 1067-1075. Stasinakis et al., 2013
- ^ Thiele B, Guenther K, Schwuger MJ (1997). "Alkylphenol ethoxylates: trace analysis and environmental behaviour". Chem Rev. 97 (8): 3247–72. doi:10.1021/cr970323m. PMID 11851490.
- ^ Pesticide Action Network North America. PANNA. Nonylphenol Etoxylates.. Accessed 9/30/2011. UK Environment Agency
- ^ Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. (2008). "Review article: Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters". Environment International. 34 (7): 1033–1049. doi:10.1016/j.envint.2008.01.004. PMID 18282600.
- ^ Minnesota Pollution Control Agency Statewide Endocrine Disrupting Compound Monitoring Study, 2007 - 2008
- ^ a b c d e f g WHO (World Health Organization). 2002. Integrated Risk Assessment: Nonylphenol Case Study. WHO/IPCS/IRA/12/04, World Health Organization, Geneva, Switzerland.
- ^ Canada, Health (2004-07-26). "ARCHIVED - Priority Substances List Assessment Report for Nonylphenol and its Ethoxylates". aem. Retrieved 2019-10-03.
- ^ Liney, Katherine E.; Hagger, Josephine A.; Tyler, Charles R.; Depledge, Michael H.; Galloway, Tamara S.; Jobling, Susan (April 2006). "Health Effects in Fish of Long-Term Exposure to Effluents from Wastewater Treatment Works". Environ Health Perspect. 114 (Suppl 1): 81–89. doi:10.1289/ehp.8058. PMC 1874182. PMID 16818251.
- ^ Asimakopoulos, Alexandros G.; Thomaidis, Nikolaos S.; Koupparis, Michael A. (2012). "Recent Trends in Biomonitoring of Bisphenol A, 4-t-octylphenol, and 4-nonylphenol". Toxicology Letters. 210 (2): 141–54. doi:10.1016/j.toxlet.2011.07.032. PMID 21888958.
- ^ a b Bechi, Nicoletta; Ietta, Francesca; Romagnoli, Roberta; Jantra, Silke; Cencini, Marco; Galassi, Gianmichele; Serchi, Tommaso; Corsi, Ilaria; Focardi, Silvano; Paulesu, Luana (2009). "Environmental Levels of Para-Nonylphenol Are Able to Affect Cytokine Secretion in Human Placenta". Environmental Health Perspectives. 118 (3): 427–31. doi:10.1289/ehp.0900882. PMC 2854774. PMID 20194071.
- ^ a b c Grün, Felix; Blumberg, Bruce (2009). "Endocrine Disrupters as Obesogens". Molecular and Cellular Endocrinology. 304 (1–2): 19–29. doi:10.1016/j.mce.2009.02.018. PMC 2713042. PMID 19433244.
- ^ "Endocrine Disruption Fact Sheet." Endocrinedisruption.org. TEDX the Endocrine Disruption Exchange, 7 Nov. 2011. Web. 25 Apr. 2014.
- ^ Gao, Q.; Horvath, T.L. (2008). "Cross-talk between estrogen and leptin signaling in the hypothalamus". Am. J. Physiol. Endocrinol. Metab. 294 (5): E817–E826. doi:10.1152/ajpendo.00733.2007. PMID 18334610. S2CID 20370652.
- ^ Masuo, Y.; Morita, M.; Oka, S.; Ishido, M. (2004). "Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain". Regul. Pept. 123 (1–3): 225–234. doi:10.1016/j.regpep.2004.05.010. PMID 15518916. S2CID 9419249.
- ^ Sakata, I.; Tanaka, T.; Yamazaki, M.; Tanizaki, T.; Zheng, Z.; Sakai, T. (2006). "Gastric estrogen directly induces ghrelin expression and production in the rat stomach". J. Endocrinol. 190 (3): 749–757. doi:10.1677/joe.1.06808. PMID 17003276.
- ^ Kim, M.S.; Yoon, C.Y.; Jang, P.G.; Park, Y.J.; Shin, C.S.; Park, H.S.; Ryu, J.W.; Pak, Y.K.; Park, J.Y.; Lee, K.U.; Kim, S.Y.; Lee, H.K.; Kim, Y.B.; Park, K.S. (2004). "The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes". Mol. Endocrinol. 18 (9): 2291–2301. doi:10.1210/me.2003-0459. PMID 15178745.
- ^ Jubendradass, R; D'Cruz, S; Mathur, P (2012). "Long-term exposure to nonylphenol affects insulin signaling in the liver of adult male rats". Hum. Exp. Toxicol. 31 (9): 868–876. doi:10.1177/0960327111426587. PMID 22076496. S2CID 38257683.
- ^ a b Vivacqua, Adele; Grazia Recchia, Anna; Fasanella, Giovanna; Gabriele, Sabrina; Carpino, Amalia; Rago, Vittoria; Maria; Di Gioia, Luisa; Leggio, Antonella; Bonofiglio, Daniela; Liguori, Angelo; Maggiolini, Marcello (2003). "The Food Contaminants Bisphenol A and 4-Nonylphenol Act as Agonists for Estrogen Receptor α in MCF7 Breast Cancer Cells". Endocrine. 22 (3): 275–84. doi:10.1385/endo:22:3:275. PMID 14709801. S2CID 24384354.
- ^ Guenther, Klaus; Heinke, Volkmar; Thiele, Bjoern; Kleist, Einhard; Prast, Hartmut; Raecker, Torsten (2002-04-15). "Endocrine disrupting nonylphenols are ubiquitous in food". Environmental Science & Technology. 36 (8): 1676–1680. Bibcode:2002EnST...36.1676G. doi:10.1021/es010199v. ISSN 0013-936X. PMID 11993862.
- ^ Raecker, Torsten; Thiele, Bjoern; Boehme, Roswitha M.; Guenther, Klaus (2011-03-01). "Endocrine disrupting nonyl- and octylphenol in infant food in Germany: Considerable daily intake of nonylphenol for babies". Chemosphere. 82 (11): 1533–1540. Bibcode:2011Chmsp..82.1533R. doi:10.1016/j.chemosphere.2010.11.065. PMID 21185059.
- ^ a b Ademollo, N.; Ferrara, F.; Delise, M.; Fabietti, F.; Funari, E. (2008). "Nonylphenol and octylphenol in human breast milk". Environ. Int. 34 (7): 984–987. doi:10.1016/j.envint.2008.03.001. PMID 18410965.
- ^ Jeannot, R.; Sabik, H.; Sauvard, E.; Dagnac, T.; Dohrendorf, K. (2002-10-18). "Determination of endocrine-disrupting compounds in environmental samples using gas and liquid chromatography with mass spectrometry". Journal of Chromatography A. 974 (1–2): 143–159. doi:10.1016/s0021-9673(02)01240-2. PMID 12458934.
- ^ Kim, Yun-Seok; Katase, Takao; Sekine, Sayaka; Inoue, Tadashi; Makino, Mitsuko; Uchiyama, Taketo; Fujimoto, Yasuo; Yamashita, Nobuyoshi (2004-02-01). "Variation in estrogenic activity among fractions of a commercial nonylphenol by high performance liquid chromatography". Chemosphere. 54 (8): 1127–1134. Bibcode:2004Chmsp..54.1127K. doi:10.1016/j.chemosphere.2003.09.024. PMID 14664841.
- ^ Bhatt, B. D.; Prasad, J. V; Kalpana, G.; Ali, S. (1992-06-30). "Separation and Characterization of Isomers of p-Nonylphenols by Capillary GC/GC-MS/GC-FTIR Techniques". Journal of Chromatographic Science. 30 (6): 203–210. doi:10.1093/chromsci/30.6.203.
- ^ Moeder, M.; Martin, C.; Harynuk, J.; Górecki, T.; Vinken, R.; Corvini, P. F. X. (2006-01-13). "Identification of isomeric 4-nonylphenol structures by gas chromatography–tandem mass spectrometry combined with cluster analysis". Journal of Chromatography A. 1102 (1–2): 245–255. doi:10.1016/j.chroma.2005.10.031. PMID 16271268.
- ^ Moeder, M.; Martin, C.; Schlosser, D.; Harynuk, J.; Górecki, T. (2006-02-24). "Separation of technical 4-nonylphenols and their biodegradation products by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry". Journal of Chromatography A. 1107 (1–2): 233–239. doi:10.1016/j.chroma.2005.12.092. PMID 16427065.
- ^ Boehme, Roswitha M.; Andries, Thomas; Dötz, Karl Heinz; Thiele, Bjoern; Guenther, Klaus (2010-08-01). "Synthesis of defined endocrine-disrupting nonylphenol isomers for biological and environmental studies". Chemosphere. 80 (7): 813–821. Bibcode:2010Chmsp..80..813B. doi:10.1016/j.chemosphere.2010.03.064. PMID 20452641.
- ^ Guether, Klaus; Kleist, Einhard; Thiele, Bjoern (2005-12-10). "Estrogen-active nonylphenols from an isomer-specific viewpoint: a systematic numbering system and future trends". Analytical and Bioanalytical Chemistry. 384 (2): 542–546. doi:10.1007/s00216-005-0181-8. PMID 16341851. S2CID 39833642.
- ^ Zhang, Haifeng; Zuehlke, Sebastian; Guenther, Klaus; Spiteller, Michael (2007-01-01). "Enantioselective separation and determination of single nonylphenol isomers". Chemosphere. 66 (4): 594–602. Bibcode:2007Chmsp..66..594Z. doi:10.1016/j.chemosphere.2006.08.012. PMID 17027900.
- ^ Zhang, Haifeng; Oppel, Iris M.; Spiteller, Michael; Guenther, Klaus; Boehmler, Gabriele; Zuehlke, Sebastian (2009-02-01). "Enantiomers of a nonylphenol isomer: Absolute configurations and estrogenic potencies". Chirality. 21 (2): 271–275. doi:10.1002/chir.20556. ISSN 1520-636X. PMID 18553459.
- ^ Acir, Ismail-Hakki; Wüst, Matthias; Guenther, Klaus (2016-05-28). "Enantioselective separation of defined endocrine-disrupting nonylphenol isomers". Analytical and Bioanalytical Chemistry. 408 (20): 5601–5607. doi:10.1007/s00216-016-9661-2. ISSN 1618-2642. PMID 27236316. S2CID 11005914.
- ^ Official Journal of the European Union: DIRECTIVE 2003/53/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 18 June 2003 amending for the 26th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (nonylphenol, nonylphenol ethoxylate and cement), July 17, 2003
- ^ a b David, A.; Fenet, H.; Gomez, E. (2009). "Alkylphenols in marine environments: distribution monitoring strategies and detection considerations". Mar. Pollut. Bull. 58 (7): 953–960. doi:10.1016/j.marpolbul.2009.04.021. PMID 19476957.
See what we do next...
OR
By submitting your email or phone number, you're giving mschf permission to send you email and/or recurring marketing texts. Data rates may apply. Text stop to cancel, help for help.
Success: You're subscribed now !
