
Alvocidib
| |
| |
| Names | |
|---|---|
| IUPAC name
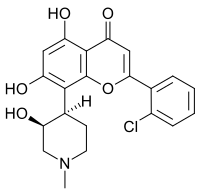
2′-Chloro-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]flavone
| |
| Systematic IUPAC name
2-(2-Chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]-4H-1-benzopyran-4-one | |
| Other names
Flavopiridol, HMR 1275, L-868275
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | Flavopiridol |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H20ClNO5 | |
| Molar mass | 401.8402 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Alvocidib (INN; also known as flavopiridol) is a flavonoid alkaloid CDK9 kinase inhibitor under clinical development by Tolero Pharmaceuticals for the treatment of acute myeloid leukemia. It has been studied also for the treatment of arthritis[1] and atherosclerotic plaque formation.[2] The target of alvocidib is the positive transcription elongation factor P-TEFb.[3][4] Treatment of cells with alvocidib leads to inhibition of P-TEFb and the loss of mRNA production.[5][6]

The compound is a synthetic analog of natural product rohitukine which was initially extracted from Aphanamixis polystachya (formerly Amoora rohituka, hence the name) and later from Dysoxylum binectariferum.[7][8]

Orphan drug
The FDA has granted orphan drug designation to alvocidib for the treatment of patients with acute myeloid leukemia.[9]

References
- ^ Sekine C, Sugihara T, Miyake S, Hirai H, Yoshida M, Miyasaka N, Kohsaka H (2008). "Successful treatment of animal models of rheumatoid arthritis with small-molecule cyclin-dependent kinase inhibitors". J. Immunol. 180 (3): 1954–61. doi:10.4049/jimmunol.180.3.1954. PMID 18209094.
- ^ Ruef J, Meshel AS, Hu Z, Horaist C, Ballinger CA, Thompson LJ, Subbarao VD, Dumont JA, Patterson C (1999). "Flavopiridol inhibits smooth muscle cell proliferation in vitro and neointimal formation In vivo after carotid injury in the rat". Circulation. 100 (6): 659–65. doi:10.1161/01.cir.100.6.659. PMID 10441105.
- ^ Chao SH, Fujinaga K, Marion JE, Taube R, Sausville EA, Senderowicz AM, Peterlin BM, Price DH (2000). "Flavopiridol inhibits P-TEFb and blocks HIV-1 replication". J. Biol. Chem. 275 (37): 28345–8. doi:10.1074/jbc.C000446200. PMID 10906320.
- ^ Chao SH, Price DH (2001). "Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo". J. Biol. Chem. 276 (34): 31793–9. doi:10.1074/jbc.M102306200. PMID 11431468.
- ^ Cheng B, Li T, Rahl PB, Adamson TE, Loudas NB, Guo J, Varzavand K, Cooper JJ, Hu X, Gnatt A, Young RA, Price DH (2012). "Functional association of Gdown1 with RNA polymerase II poised on human genes". Mol. Cell. 45 (1): 38–50. doi:10.1016/j.molcel.2011.10.022. PMC 3259526. PMID 22244331.
- ^ Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA (2010). "c-Myc regulates transcriptional pause release". Cell. 141 (3): 432–45. doi:10.1016/j.cell.2010.03.030. PMC 2864022. PMID 20434984.
- ^ Harmon, AD; Weiss, U; Silverton, JV (1979). "The structure of rohitukine, the main alkaloid of Amoora rohituka (syn.Aphanamixis polystachya) (Meliaceae)". Tetrahedron Lett. 20 (1): 721–724. doi:10.1016/S0040-4039(01)93556-7.
- ^ Lakdawala, AD; Shirole, MV; Mandrekar, SS; Dohadwalla, AN (1988). "Immunopharmacological potential of rohitukine: a novel compound isolated from the plant Dysoxylum binectariferum". Asia Pac J Pharmcol. 3 (1): 91–98.
- ^ "FDA grants orphan drug status to Alvocidib for AML".

See what we do next...
OR
By submitting your email or phone number, you're giving mschf permission to send you email and/or recurring marketing texts. Data rates may apply. Text stop to cancel, help for help.
Success: You're subscribed now !
