
Tiazofurin
 | |
| Clinical data | |
|---|---|
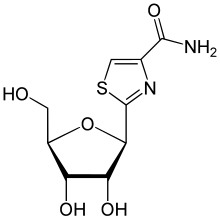
| Other names | 2-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-thiazole-4-carboxamide |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H12N2O5S |
| Molar mass | 260.26 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tiazofurin is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. Tiazofurin and its analogues were under investigation for potential use in the treatment of cancer,[1] though side effects such as pleuropericarditis and a flu-like syndrome precluded further development. They also show antiviral effects and may be reevaluated as potential options in the treatment of newly emerging viral diseases.[2]

Synthesis

The treatment of 1-O-Acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose [6974-32-9] (1) with trimethylsilyl cyanide gives 2,3,5-Tri-O-benzoyl-beta-D-ribofuranosyl cyanide [23316-67-8] (2). Treatment with hydrogen sulfide led to (2R,3R,4R,5R)-2-((Benzoyloxy)methyl)-5-carbamothioyltetrahydrofuran-3,4-diyl dibenzoate, PC10907289 (3). Cyclization with Ethyl bromopyruvate [70-23-5] (4) led to 2-(2,3,5-Tri-O-benzoyl-beta-D-ribofuranosyl)-4-thiazolecarboxylic Acid Ethyl Ester [60084-09-5] (5). Removal of the protecting groups with sodium methoxide afforded 2-beta-D-Ribofuranosyl-4-thiazolecarboxylic Acid Ethyl Ester [95936-53-1] (6). Amide-ester interchange by treatment with dry ammonia completed the synthesis of Tiazofurin (7).

References
- ^ Popsavin M, Torović L, Svircev M, et al. (2006). "Synthesis and antiproliferative activity of two new tiazofurin analogues with 2'-amido functionalities". Bioorg. Med. Chem. Lett. 16 (10): 2773–6. doi:10.1016/j.bmcl.2006.02.001. PMID 16495053.
- ^ De Clercq E (March 2016). "C-Nucleosides To Be Revisited". Journal of Medicinal Chemistry. 59 (6): 2301–11. doi:10.1021/acs.jmedchem.5b01157. PMID 26513594.
- ^ Eastland, G.; Tiazofurine. Drugs Fut 1985, 10, 4, 304.

See what we do next...
OR
By submitting your email or phone number, you're giving mschf permission to send you email and/or recurring marketing texts. Data rates may apply. Text stop to cancel, help for help.
Success: You're subscribed now !
